Background or Introduction:
Gemtuzumab Ozogamicin (GO), a therapeutic antibody targeting CD33, gained FDA approval in 2017 for the treatment of patients with Acute Myeloid Leukemia (AML). Biomarkers to predict GO response were historically based on multiple indicators: CD33 expression levels, favorable cytogenetics and specific genetic alterations (NPM1, FLT3-internal tandem duplications, other mutations). Moreover, comprehensive analyses including stemness expression (LSC17 score) and molecularly derived scores such as the CD33_PGx6_Score (a composite score derived from six CD33 SNP of prognostic significance) have further refined the biomarkers to identify potential responders (Fenwarth et al, nt. J. Mol. Sci. 2020, 21, 5626). However, due to its toxicity profile and elevated cost, the identification of robust biomarkers of GO response in AML remains a challenge to optimize its therapeutic efficacy and worldwide clinical adoption.
Methods:
In this study, we utilized an innovative ex-vivo functional platform of Patient Micro Avatars (PMAs) developed at OncoPrecision (García et al, Blood 2022 140 (Supplement 1): 13027-13028) to assess the response to GO in a small cohort of AML patients. Patient-Derived Cells (PDCs) were obtained from five newly diagnosed AML patients and GO activity was assessed using high-throughput flow cytometry. The correlation between CD33 expression and the ex-vivo response to GO was also examined.
Results:
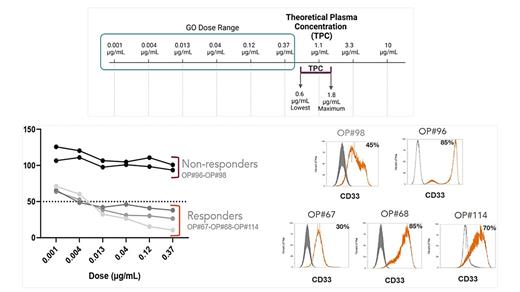
Our findings revealed a clear differential response to GO in AML patients, with contrasting outcomes observed within a dose-range that does not exceed its theoretical plasma concentration (Figure - Upper Panel). Interestingly, despite significant variations in CD33 expression levels among the evaluated patients, no obvious association was found between CD33 expression and the ex-vivo response to GO (Figure - Lower Panel).
Conclusions:
This study presents an innovative approach with the potential to predict GO responders versus non-responders in AML. A single phenotypic biomarker overcomes the need to determine CD33 expression levels in each patient or to assess the presence of specific polymorphic variants, as well as additional molecular biomarkers. Patient Micro Avatars present a compelling alternative to comprehensively identify patients who are most likely to benefit from GO treatment.
References:
1. Fenwarth L, ... Duployez N. Biomarkers of Gemtuzumab Ozogamicin Response for Acute Myeloid Leukemia Treatment. Int J Mol Sci. 2020 Aug 6;21(16):5626. doi: 10.3390/ijms21165626. PMID: 32781546; PMCID: PMC7460695.
2. Alejandra Garcia, ... Gastón Soria; Ex-Vivo Testing Using Patient Micro Avatars (PMAs) Predicts Clinical Response in Acute Leukemias. Blood 2022; 140 (Supplement 1): 13027-13028. doi: https://doi.org/10.1182/blood-2022-164847
Disclosures
Garro:OncoPrecision: Current Employment. Ridano:OncoPrecision: Consultancy, Honoraria. Buffa:OncoPrecision: Current Employment. Cavallo:OncoPrecision: Current Employment. Conrrero:OncoPrecision: Current Employment. Ferreira:OncoPrecision: Current Employment. Piaggio:OncoPrecision: Current Employment. Zaki:OncoPrecision: Current Employment, Current equity holder in private company, Current holder of stock options in a privately-held company. Gatti:OncoPrecision: Current Employment, Current equity holder in private company. Llorens:OncoPrecision: Current Employment, Current equity holder in private company. Soria:OncoPrecision: Current Employment, Current holder of stock options in a privately-held company.